
Balancing Chem Equations WS1 PDF
Chemical equations and formulas are a vital part of learning chemistry from 11‒19. Being able to use them correctly is part of what it means to be a chemist. Formulas and equations allow chemists to communicate chemical knowledge efficiently. Students should understand that chemical formulas:
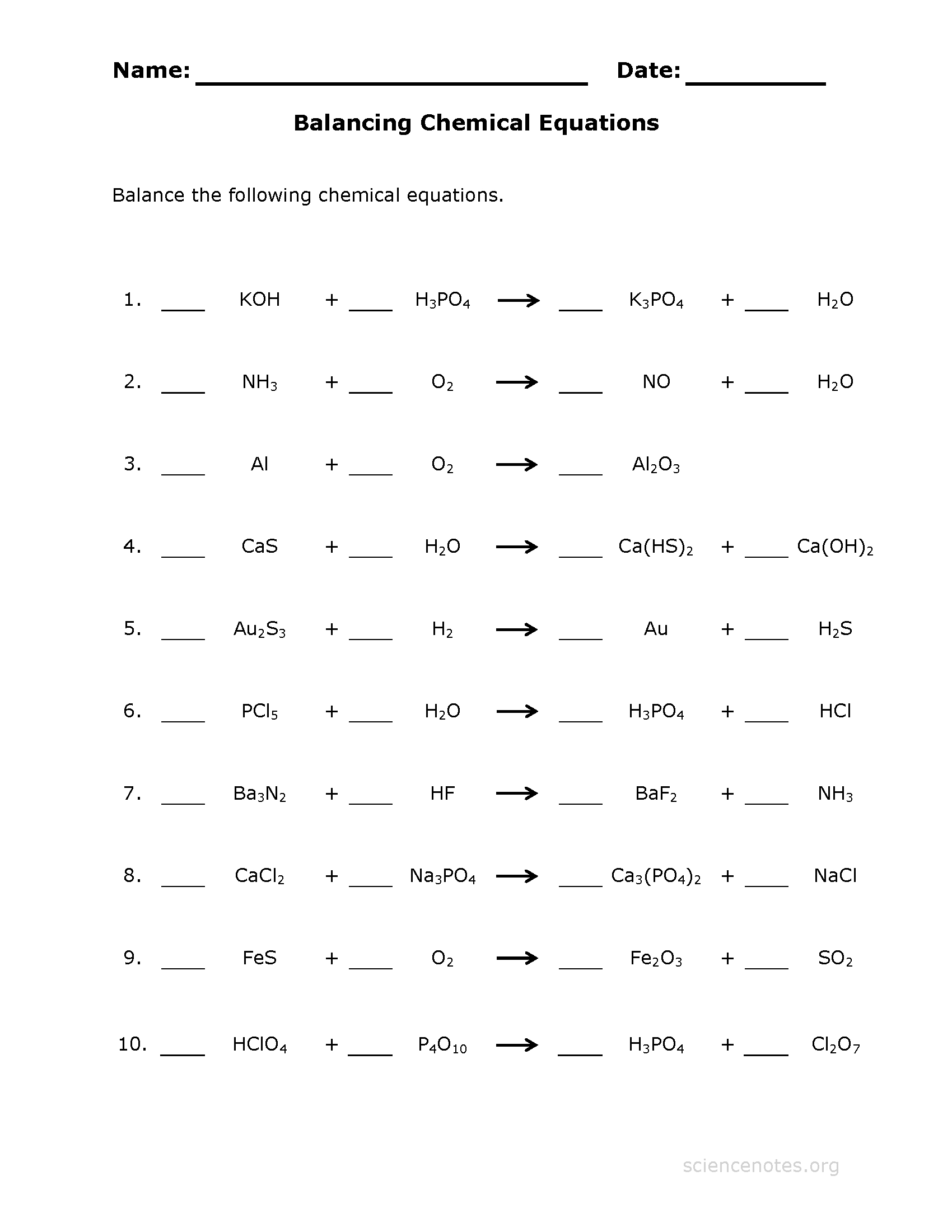
Balancing Chemical Equations Practice Worksheet —
A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substance), the formulas of the participants, the phases of the participants (solid, liquid, gas), and the amount of each substance.

Balancing Chemical Equations Mr. Durdel's Chemistry WMS Chemistry classroom, Chemical
Part of Chemistry (Single Science) The nature of substances and chemical reactions Remove from My Bitesize Balancing equations and calculations Word equations are useful to show which.

Chemistry+Equation+Sheet.pdf Enthalpy Chemical Reactions
CHAPTER1 C onsider the following situations of daily life and think what happens when - milk is left at room temperature during summers. an iron tawa/pan/nail is left exposed to humid atmosphere. grapes get fermented. food is cooked. food gets digested in our body. we respire.
HelpWork balancing chemistry equations
1 atom Fe 2 atoms O Right side: 2 atoms Fe 3 atoms O The balancing of the equation is accomplished by introducing the proper number or coefficient before each formula. To balance the number of O atoms, write a 3 in from of the O2 and a 2 in front of the Fe2O3: Fe + 3 O2 → 2 Fe2O3

Exemplary 100 Examples Of Chemical Equations Balanced Molecular Equation Calculator
Chapter 11. Chemical Equations 11.1. ChemicalEquation Concepts Under the right circumstances substances change into newsubstances. Suchchanges are calledchemical transformations,orreactions.The original substances are calledreac- tants,the substances resulting from the change are calledproducts.Chemical reactions reveal the basic properties of substances that form the heart of chemistry.

List of chemical formula or common name of all compound Brainly.in Chemical
Chemistry Basic Formulas & Equations Reference. The complete list of chemistry basic equations & formulas cheat sheet for PDF download to help users to use them offline to learn or workout how to execute or solve the various calculations of normality, molarity, molality, enthalpy, entropy, gas, energy, equilibrium and much more.
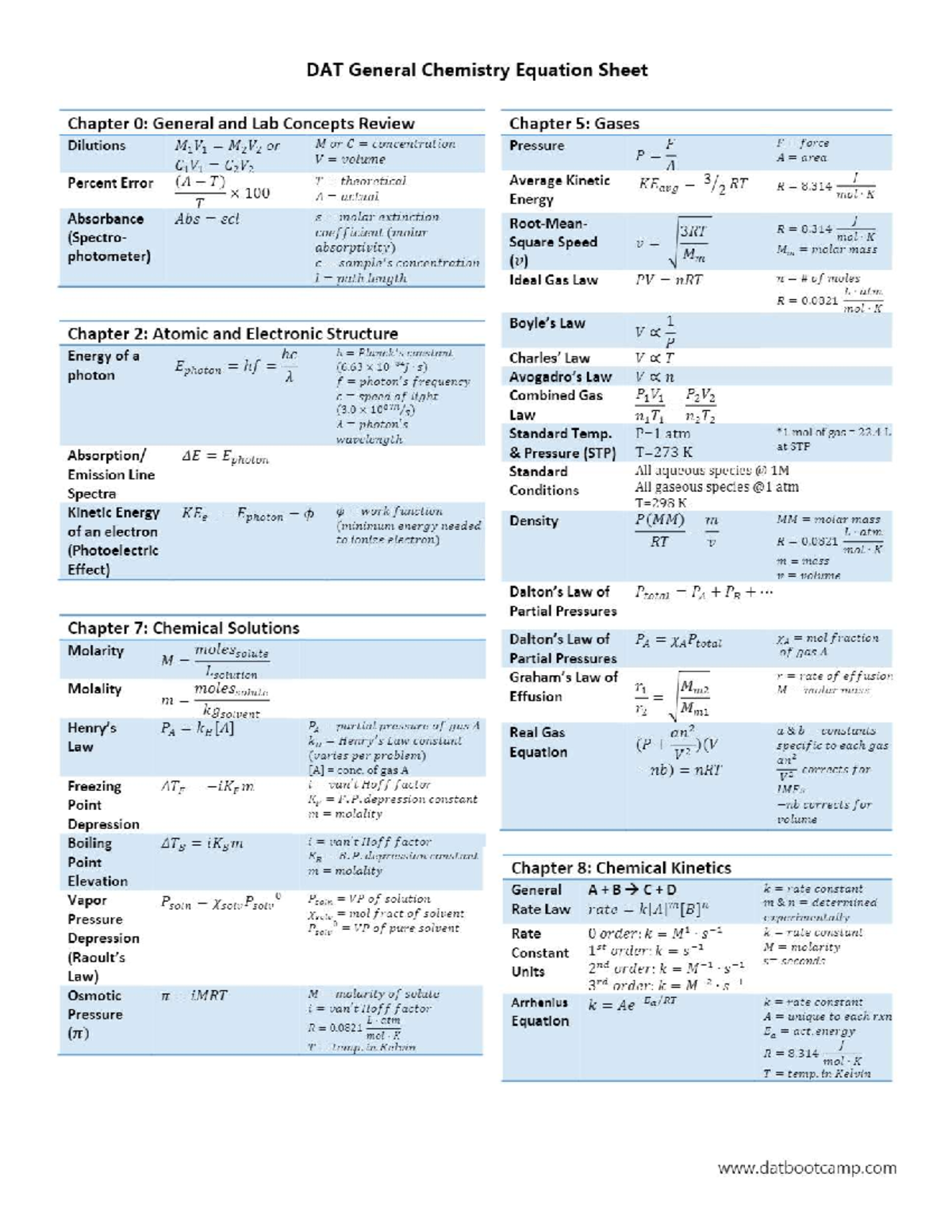
Chem equation sheet for chemistry class Eng Tech 1Ch3 McMaster StuDocu
The general equation for a chemical reaction is: As to why water is produced as a gas, it may be observed that the water is produced as a result of an explosion as shown in Figure 7.3.1 7.3. 1. At the elevated temperature of the explosion, water exists as a gas and not as a liquid.
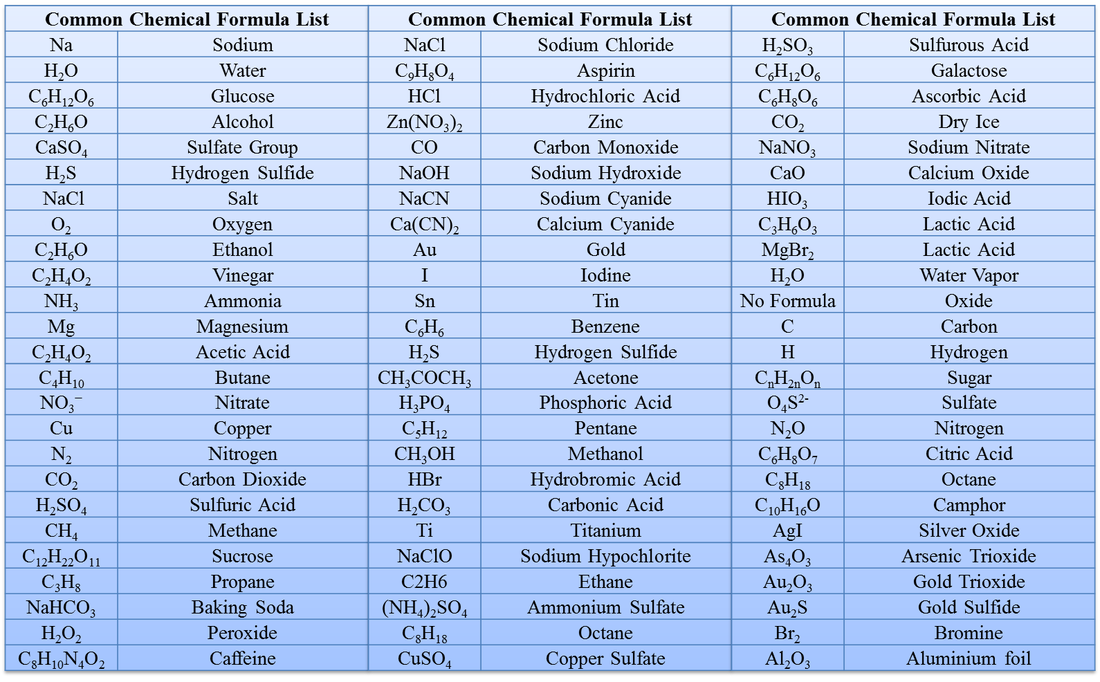
Chemical Formula ( रासायनिक नाम और सूत्र ) List, Table, Chart And PDF
Chemical Formula Definition: An expression which states the number and type of atoms present in a molecule of a substance. Chemical formulas such as HClO4 can be divided into empirical formula, molecular formula, and structural formula.

Student Exploration Balancing Chemical Equations Conservation Of Mass Lab Worksheets Teaching
Microsoft Word - Chapter 6.doc. 82. Returning to our earlier, mistake free equation: CH4 + O2. →. CO2 + H2O. We are ready for the second absolute requirement: "The number of atoms of each. element must be the same on both sides of the equation.". In our equation, we have.

WORKSHEET (chemical equations).pdf Chemical Reactions Unit Processes
CH4 +Cl2 → CCl4 + HCl (5.3.5) (5.3.5) CH 4 + Cl 2 → CCl 4 + HCl. Step 2: Count the number of each atom or polyatomic ion on both sides of the equation. Reactants 1Catom 4H ions 2Clatom Products 1Catom 1H ions 5Clatoms Reactants Products 1 C atom 1 C atom 4 H ions 1 H ions 2 Cl atom 5 Cl atoms.
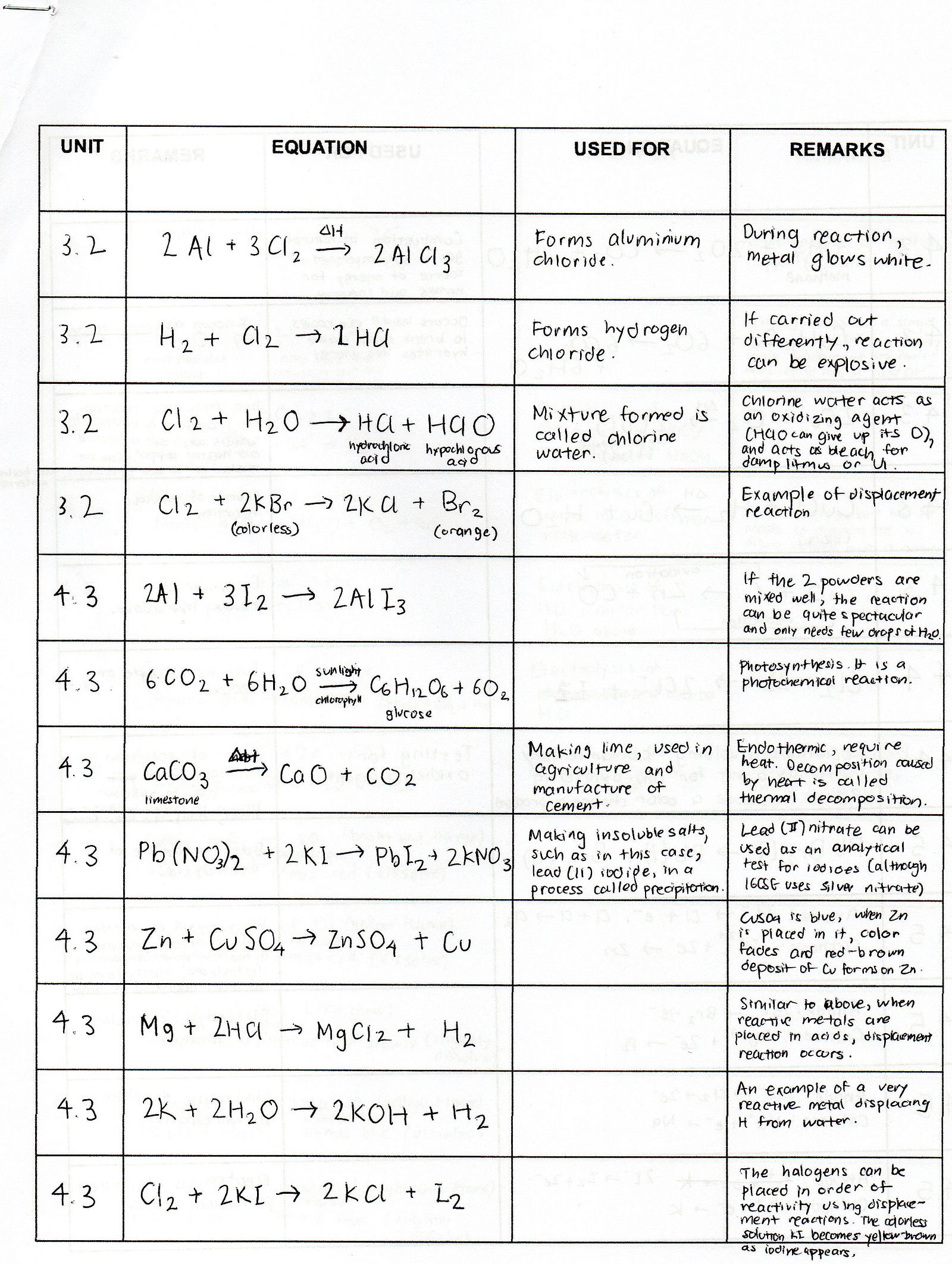
Chemistry Formulas And Equations Review Tessshebaylo
A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound (s)—the reactants —on the left and the final compound (s)—the products —on the right, separated by an arrow.

Chemical Equations PDF
chemical reactions when the names of the reactants and products are specified; 1.5.8 write balanced ionic equations for reactions covered in this specification; 1.5.9 write half equations for reactions covered in this specification; 1.5.10 demonstrate knowledge and understanding that in chemical equations the three states of matter

Chemistry Formula Sheet AP Chemistry Ion Sheet Chemistry Pinterest Ap chemistry
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.
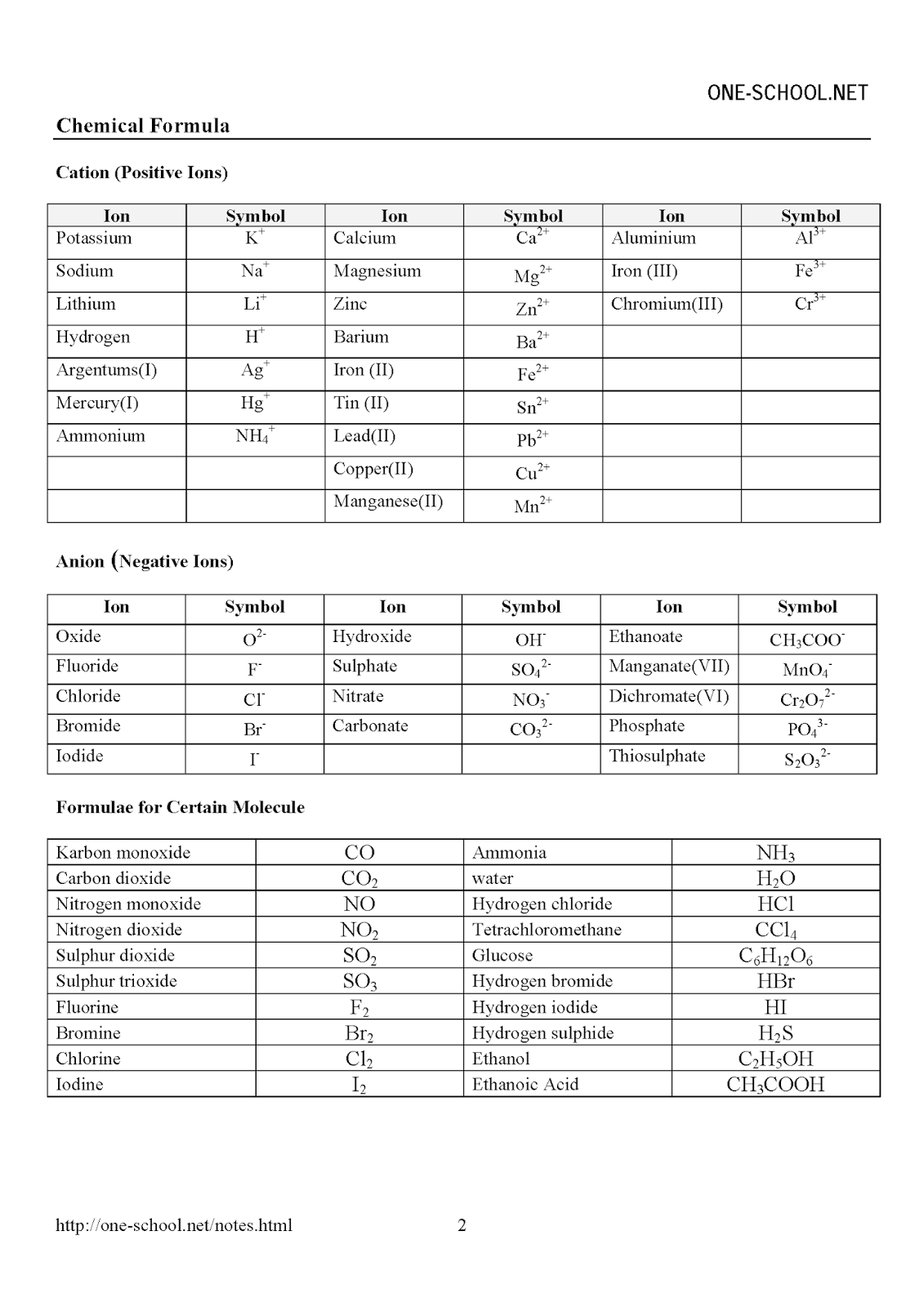
SPM Form 4 Chemistry Formulae List SPM Chemistry
4.4 Chemical changes 43 4.5 Energy changes 51 4.6 The rate and extent of chemical change 55 4.7 Organic chemistry 61 4.8 Chemical analysis 70 4.9 Chemistry of the atmosphere 75 4.10 Using resources 80 4.11 Key ideas 89 5 Scheme of assessment 91 5.1 Aims and learning outcomes 91 5.2 Assessment objectives 92 5.3 Assessment weightings 93

How to Write a Chemical Equation (with Pictures) wikiHow
CHAPTER 1 WHAT ARE CHEMICAL ENGINEERING AND BIOENGINEERING? 3 1.1 Introduction 3 1.2 A Brief History of Chemical Engineering 4. 7.2 Real Gases: Equations of State 366 7.3 Real Gases: Compressibility Charts 377 7.4 Real Gas Mixtures 384 CHAPTER 8 MULTIPHASE EQUILIBRIUM 411